Propene undergoes addition polymerization to produce poly(propene), often known as polypropylene, which is one of the most versatile thermoplastic polymers available commercially. Mixtures of propene and other monomers form a wide range of important co-polymers. Industrial uses of Polypropylene
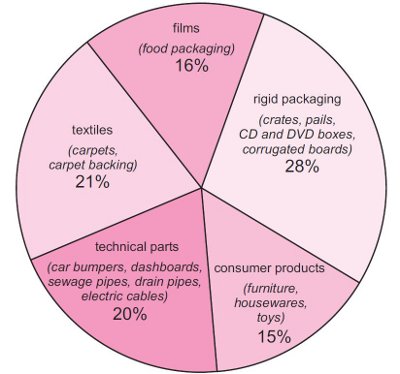
Poly(propene) is processed into film, for packaging and into fibres for carpets and clothing. It is also used for injection moulded articles ranging from car bumpers to washing up bowls, and can be extruded into pipe (Figure 1).
Figure 1 Uses of poly(propene).
Materials suitable for a much wider range of applications can be made by compounding poly(propene) with, for example, fillers and pigments, and elastomers.
Poly(propene) has remarkable properties, making it suitable to replace glass, metals, cartons and other polymers.
These properties include:
- low density (weight saving)
- high stiffness
- heat and U.V resistance
- chemical inertness
- steam barrier properties (food protection)
- good transparency
- good impact/rigidity balance
- stretchability (film and fibre applications)
- good hinge property (for example where a lid and box are made together, fsuch as DVD cases)
- high gloss (appearance)
- easy to weld (design)
- recyclability friendly
The majority (ca 60% of the total produced) of poly(propene) is produced as a homopolymer. Co-polymers are discussed below.
Poly(propene) is one of the lightest thermoplastics (density 0.905 g cm-3). It has a melting point of 440 K and a crystallinity of ca 50-60%. The polymer, unlike poly(ethene), is transparent.
Structure of the polymer
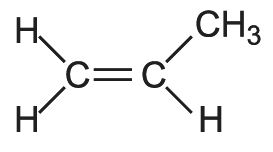
The propene molecule is asymmetrical,
and, when polymerized, can form three basic chain structures dependent on the position of the methyl groups: two are stereoregular (isotactic and syndiotactic) and the third does not have a regular structure and is termed atactic as shown diagrammatically below:
The 'one handed' structure of isotactic poly(propene) causes the molecules to form helices. This regular form permits the molecules to crystallize to a hard, relatively rigid material, which, in its pure form, melts at 440 K.
The syndiotactic polymer, because of its regular structure, is also crystalline.
Atactic chains are completely random in structure and consequently they do not crystallize. High molecular mass atactic poly(propene) is a rubber-like material.
Commercial poly(propene) is a predominantly isotactic polymer containing 1-5% by mass of atactic material.
Manufacture of poly(propene) (polypropylene)
Poly(propene) is produced from propene. Propene is produced in large quantities from gas oil, naphtha, ethane and propane.
Parallel to this, there are several methods being developed to produce bio-based poly(propene) (bio-based polypropylene) via bio-based-propene.
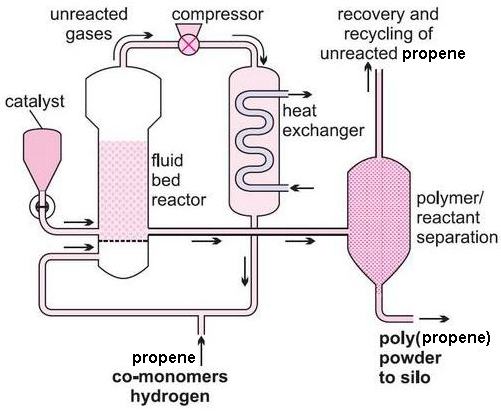
The polymer is separated from the gaseous propene and hydrogen using cyclones and the unreacted gas is recycled.
Both processes can be operated continuously and use 'stereospecific' Ziegler-Natta catalysts to effect the polymerization. The catalyst remains in the product and needs to be destroyed using water or alcohols, before the polymer is converted into pellets.
Both bulk and gas phase processes have virtually eliminated gaseous and aqueous effluents by the use of high activity catalysts, resulting in low residues in the final polymer.
(b) Using a metallocene as catalyst
Metallocenes are being increasingly used as catalysts for the production of poly(ethene) (mLLDPE) and poly(propene).
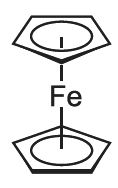
Metallocenes are strictly defined as molecules which have a transition metal atom bonded between two cyclopentadienyl ligands which are in parallel planes. Ferrocene is a particularly well known example:
However, the term is now used more widely to include other ligands related to cyclopentadienyl. One such metallocene is based on zirconium:
Zirconium has an oxidation state of 4 and is bonded to two indenyl ligands (a cyclopentadienyl ligand fused to a benzene ring). They are joined by two CH2 groups. In conjunction with an organoaluminium compound, it acts as a catalyst for the polymerization of alkenes such as ethene and propene. The specific orientation of the zirconium compound means that each propene molecule, for example, as it adds on to the growing polymer chain is in the same orientation and an isotactic polymer is produced.





 Poly(propene), (Polypropylene)
Poly(propene), (Polypropylene)